|
The first in the world! The joint detection of IgA / IgM / IgG antibody of new coronavirus, the exclusive product of Zhongjian Antai, is emerging!时间:2020-03-25 The novel coronavirus outbreak occurred in the Spring Festival in 2020, with a way of "a battle against the pneumonia epidemic infected by Novel Coronavirus suddenly started across the country". At the present, novel coronavirus infection has ravaged the world, while the number of infections grows with each passing day. At present, the novel coronavirus detection product for immunization domestic and international mainly includes a total antibody; IgM and IgG combined detection reagents, whereas the novel coronavirus specific IgA antibody detection is in a vacancy state. The world's first detectable COVID-19 specific IgA antibody product is now available. The coronavirus (COVID-19) combined IgA/IgM/IgG antibody rapid test (colloidal gold method), which is newly launched by Beijing Zhongjian Antai Diagnostic Technology Co., Ltd., can detect the antibodies of the new crown virus (COVID-19) specific IgM, IgA and IgG. The S1 protein and S2 protein of the viruses are labeled, and the freeze-drying process is adopted to ensure that the product has high sensitivity and specificity, filled will be coronavirus rapid immune diagnosis of multiple antibodies joint gap in the market. At present, the kit has obtained the European Union CE access qualification. As the first manufacturer in the world to develop a new coronavirus IgA / IgM / IgG antibody joint detection, Zhongjian Antai has 10 years of tomographic technology research and development experience, ISO13485 system certification, stable and reliable production process, which will provide the latest weapons for global epidemic prevention and clinical accurate detection.
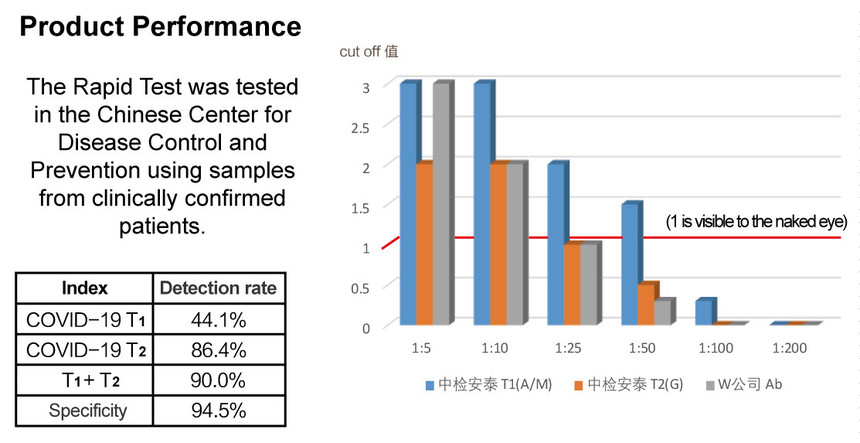
Novel coronavirus reagents round the clock were launched in January 2020. The new type of the coronavirus (COVID-19) combined IgA/IgM/IgG antibodies rapid test (colloidal gold) was completed in February 2020, with the help of the researchers worked day and night to finish the research and development work, the sensitivity and specificity are now evaluating products have reached a satisfactory result, start working for effective conversion enlarge production capacity. COVID-19 IgA/IgM/IgG antibody combination detection reagent can be used in both the acute infection stage and the recovery stage of patients and can be effectively complementary with nucleic acid detection reagent which is highly sensitive to the early infection stage, without direct interpretation by instruments. In Wuhan city, the feedback results from the evaluation of samples from 59 clinically confirmed patients used by the China CDC virus showed that the positive detection rate of novel Coronavirus (COVID-19) IgA/IgM/IgG combined detection kit produced by Zhongjian Antai reached 90%. The company's research and development laboratory used random samples collected before the outbreak of the outbreak test, reagent specificity of 94.5%.
A new coronavirus antibody test reagent of a listed company W was used to compare the detection of the coronavirus (COVID-19) combined IgA/IgM/IgG antibodies rapid test of Zhongjian Antai. The samples of the same coronavirus infected patients were detected and gradient dilution verification was conducted to compare the difference of sensitivity level between the two products. Through comparison, it is confirmed that the product sensitivity of Beijing Zhongjian Antai Diagnostic Technology Co., Ltd. is higher than that of listed W company, meeting the clinical use of infected or suspected patients in the auxiliary screening.
· It is the first in the world to combine the detection of IgA + IgM + IgG antibodies, which can effectively diagnose early and present infection and distinguish the previous infection. · High sensitivity; detection of IgA antibody can improve the positive detection rate, compared with the same reagent. · Several types of samples; either the whole blood, serum, or plasma can be used for detection, without pre-treatment. · Single independent packaging, no need for any equipment, short testing time, suitable for on-site screening. · The kit is stored at room temperature without refrigeration and cold chain transportation.
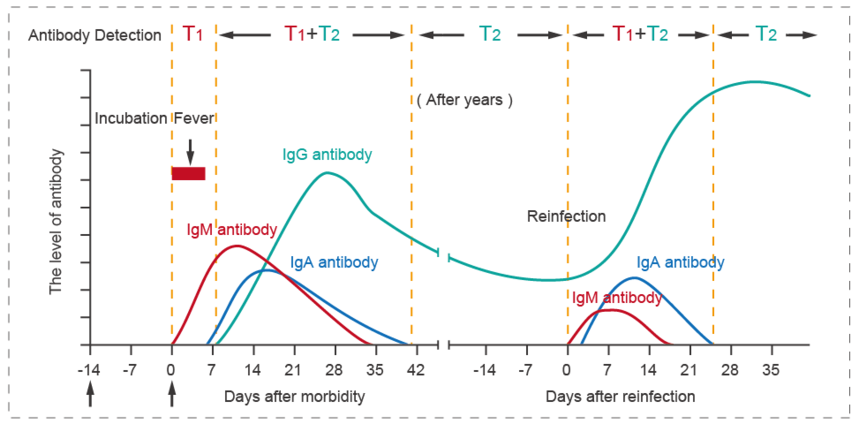
The rapid detection of IgA / IgM / IgG antibodies can be used to evaluate the development of the disease course. T1 refers to the test results of IgA and/or IgM, the positive results indicate that it may be a recent infection, which can be used to assist in the diagnosis of early and present infection of COVID-19; T2 refers to the test results of IgG, the single positive results of IgG indicate that there may be infection for a while, which can be used to assist in the diagnosis of mid-late or previous infection of COVID-19. The detection of IgA and IgM antibody of new coronavirus can supplement the diagnosis of nucleic acid negative patients with new coronavirus infection, and also show a good value for the diagnosis of asymptomatic patients with infection; focusing on early diagnosis, reducing the window period as much as possible, has an important research value for the early diagnosis of suspected cases.
The reagent has been sent to South Korea, Turkey, Iraq, Iran, Indonesia, Pakistan, Philippines, Germany and other countries. As a member of the global medical industry, Zhongjian Antai is willing to make due contributions to the global epidemic prevention.
Welcome to inquire! Website: www.bjzjat.com Phone: 010-65426941 Mailbox: zjatit@sina.com The coronavirus (COVID-19) combined IgA/IgM/IgG antibodies rapid test, an exclusive product of Zhongjian Antai, has been reported by in vitro diagnostic network and CACLP in vitro diagnostic information and other reports, with the reading capacity and reprint capacity reaching tens of thousands of people, which has attracted a lot of attention. For details, please click the links below: https://mp.weixin.qq.com/s/oD4KIWgq8IeV0gkfX1ApGg https://mp.weixin.qq.com/s/_56d0Nl8fLm4bZWqXPhCag
Founded in 2010, Beijing Zhongjian Antai Diagnostic Technology Co., Ltd. is a subsidiary of Huawei Tianhe Group. It is based on the research, development, registration, production and sales of in vitro diagnostic reagent (IVD). Aiming at the immunodiagnostic market and POCT market, we strive to expand the new application and popularization of in vitro diagnostic products in the medical industry. For the majority of medical workers to help determine what kind of medical treatment patients need and guide the treatment of drugs, provide necessary medical testing products. The company's product research and development and planning has a clear direction, the product is easy to operate, intuitive results, save time and effort, economical and affordable essential characteristics. In the process of product research and development, the inherent advantages of products should be vigorously explored to meet the booming demand related to POCT market and immune diagnosis market. Maintain the excellent inherent performance of the product and the company's technical advantages in the industry.
|