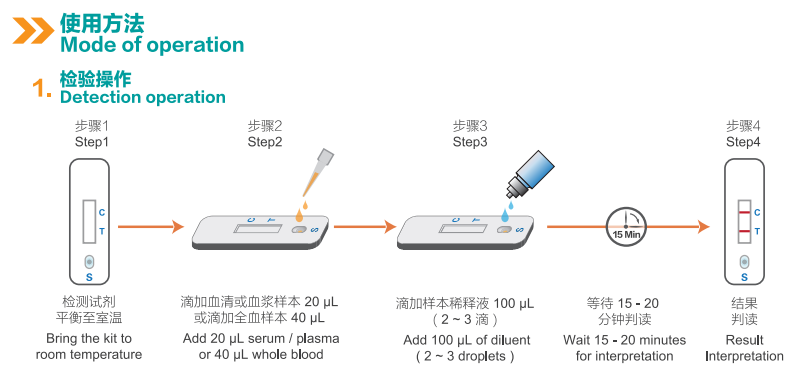
|
Corona Virus (COVID-19) Neutralizing Antibody Detection Kit (Colloidal Gold)Significance The immune system will produce corresponding antibodies after being stimulated by the virus. Some antibodies can bind to the neutralizing epitope of the virus particles, making the virus lose the ability to bind to the receptor, preventing the virus from infecting cells, so as to "neutralize" the virus. These antibodies are called neutralizing antibodies. The detection of neutralizing antibody of novel coronavirus can reflect the individual's recovery and the effect of vaccine, but the traditional virus neutralization experiment needs to use live virus and cells, and has high requirements for laboratory safety and operator skills. This time-consuming and demanding traditional neutralization detection method does not apply to COVID-19 with fast transmission speed and wide coverage. Therefore, the kit provides a simple, time-consuming and convenient auxiliary diagnostic method for the detection of of neutralizing antibody against neocoronavirus. Product features 1.No test equipment, visual interpretation. 2.Test results within 15- 20 minutes. 3.Complete sample types: serum, plasma and whole blood. 4.The operation is simple and fast. 5.Room temperature storage without cold chain.
Notice 1.The test must be done at temperature between 18 - 30C. 2.The test results can only show the level of neutralizing antibody in the sample, and can not accurately quantify the neutralizing antibody titer. 3.The rapid test should be sealed and kept in dry place and should be used as soon as the packing is opened. 4.The results of rapid test are only for dlinical reference and should not be the only basis for clinical diagnosis and treatment. 5.Waste samples and test should be treated as potential infectious agents. 6.The result should be read strictly within a time limit of 15-20 minutes. 7.This reagent can only be used to detect the level of neutralizing antibody after vaccination or recovery from infection, and cannot be used to evaluate the effectiveness of antiviral protection. |